Abstract
Background
Central nervous system (CNS) relapse is a complication that impacts approximately 2-6% of patients with diffuse large B-cell lymphoma (DLBCL). Intrathecal (IT) prophylaxis with methotrexate and cytarabine is often administered to patients with DLBCL to lower the rates of CNS relapse, although its benefit has not been well described. Multiple prognostic models, including the CNS-international prognostic index (IPI), have been developed to identify patients with high risk for CNS relapse (Hematol Oncol 2013;31:96-150, Ann Oncol 2002;13[7]:1099-1107, Ann Hematol 2016;95:1661). We conducted a single-center study to evaluate indices predicting CNS relapse, explore patterns of use of CNS prophylaxis, and describe patient outcomes based on whether they received IT prophylaxis.
Methods
We reviewed patients diagnosed with DLBCL from 2009 to 2016 at Emory Healthcare and collected data on risk factors for CNS relapse as well as utilization of CNS prophylaxis administered based on individual physician preference. Patients were stratified into high or low-risk categories for CNS relapse based on risk factors at the time of diagnosis, including those used in the CNS-IPI and similar risk models. Patients were considered high-risk if they presented with testicular involvement, double-hit lymphoma, or had 4 or more of the following: extranodal sites >1, elevated lactate dehydrogenase (LDH), retroperitoneal involvement, age >60, low albumin, stage 3 or 4 disease, and performance status (PS) >1. Only 3 of the above factors needed to be present if all were one of the following: extranodal sites >1, low albumin, stage 3 or 4, and kidney/adrenal involvement. The primary outcome was to compare the CNS relapse rate in high-risk patients who received IT prophylaxis to patients who did not. Secondary outcomes included comparing CNS relapse rates in those who received IT prophylaxis to those without prophylaxis in all study patients and in low-risk patients. Additionally, we evaluated rates of adverse effects associated with IT prophylaxis.
Results
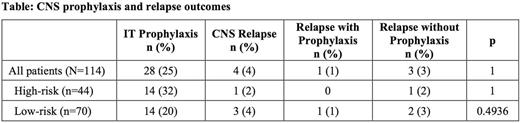
One-hundred fourteen patients were stratified into high (n=44, 39%) or low-risk (n=70, 61%) categories for CNS relapse. High-risk patients were younger than low-risk patients (median age 58 vs. 66) and more likely to present with stage 3 or 4 disease, extranodal sites >1, elevated LDH and albumin, PS >1, retroperitoneal involvement, and double-hit lymphoma. Twenty-eight patients (14 high-risk and 14 low-risk) received IT prophylaxis. Eight patients received methotrexate as their only IT chemotherapy and 20 received a combination of methotrexate and cytarabine. Only 4 of 114 patients experienced a CNS relapse, including 1 in the high-risk group and 3 in the low-risk group. Among the 14 high-risk patients who received IT prophylaxis, no patient experienced CNS relapse compared with 1 of 30 high-risk patients without prophylaxis (Table). One of the 14 low-risk patients who received prophylaxis experienced relapse. Given the small number of CNS relapses in the study, there was no statistical difference in relapse incidence between patients with high- or low-risk disease or for patients with or without IT prophylaxis. Three patients experienced significant adverse effects, leading to discontinuation of planned prophylaxis in 2 patients.
Conclusions
Our results showed no significant difference in CNS relapse rates between patients with or without IT prophylaxis regardless of risk, albeit with a relatively small sample size. The percentage of low-risk vs high risk (20% vs. 32%) patients who received IT prophylaxis was similar, thus indicating that treating physicians in this study used criteria other than the CNS-IPI to assess risk for CNS relapse. Our observation that many high-risk patients did not receive prophylaxis while many low-risk patients received prophylaxis emphasizes the need for a standardized approach. Given the low rates of CNS relapse in DLBCL, risk stratification and IT prophylaxis appear to have limited impact on outcomes in most cases. Larger prospective trials are needed to improve identification of patients at highest risk for CNS relapse.
Cohen: Infinity: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Research Funding; Takada: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; LAM Therapeutics, Inc: Research Funding; Bioinvent: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Flowers: Onyx: Research Funding; Clinical Care Options: Research Funding; Burroughs Welcome Fund: Research Funding; Infinity: Research Funding; National Institutes Of Health: Research Funding; Celgene: Consultancy, Research Funding; Bayer: Consultancy; Janssen Pharmaceutical: Research Funding; Abbvie: Consultancy, Research Funding; Educational Concepts: Research Funding; National Cancer Institute: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Millennium/Takeda: Research Funding; Research to Practice: Research Funding; TG Therapeutics: Research Funding; V Foundation: Research Funding; OptumRx: Consultancy; Acerta: Research Funding; Seattle Genetics: Consultancy; Spectrum: Consultancy; Prime Oncology: Research Funding; Genentech/Roche: Consultancy, Research Funding; Gilead: Consultancy; Eastern Cooperative Oncology Group: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal